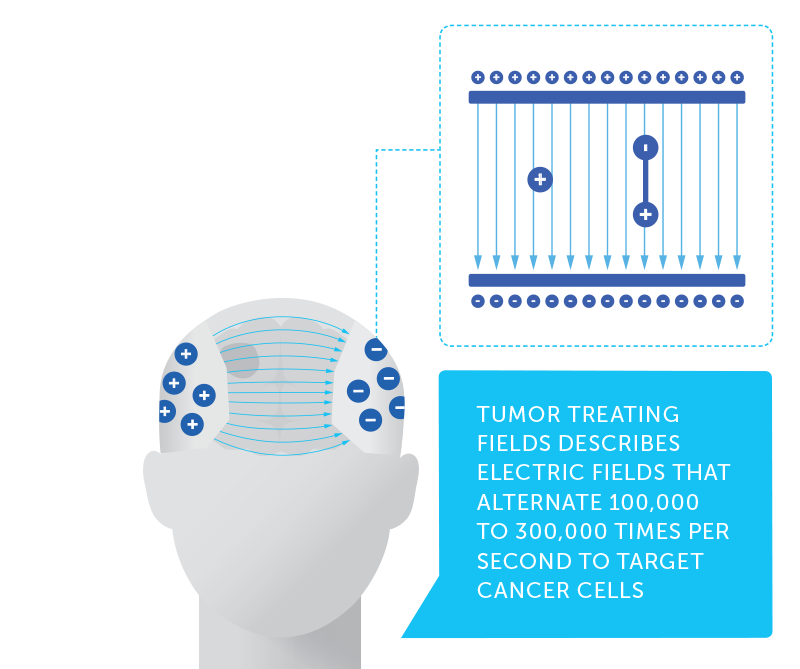
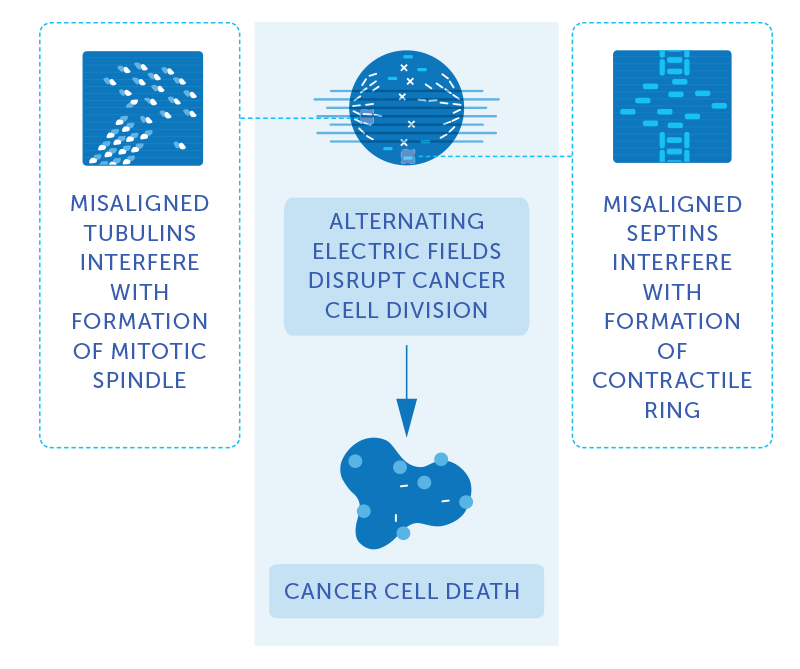
Professor Ignace Vergote has dedicated his 32-year career to investigating treatment options and striving to improve survival in gynecologic cancers. Among gynecologic cancers, ovarian cancer is the deadliest, representing the fifth most common cause of cancer death among women in the United States. Five-year survival in ovarian cancer has slowly increased from 34 percent in 1975 to 47 percent today.
“We are making progress little by little, step by step,” said Professor Vergote, Chairman of the Department of Obstetrics and Gynaecology and Gynaecologic Oncology at the Catholic University of Leuven, European Union.
Although more women are living longer with ovarian cancer today than several decades ago, 70 percent of patients will have a recurrence and most will eventually die from the disease.
“As an oncologist, you mean a lot to patients,” he said. “Many times, you can’t cure them, but you can give them a longer life with acceptable quality of life.”
To date, Professor Vergote has authored more than 800 papers in gynecologic cancer research and his work was cited more than 40,000 times. He conducts 20 clinical trials in ovarian cancer per year and was an investigator in Novocure’s phase 2 pilot study in recurrent ovarian cancer, INNOVATE.
“There are few possibilities to treat these patients,” he said. “We must continue to investigate treatment options that may potentially improve survival in this difficult to treat disease.”
Professor Vergote became interested in Tumor Treating Fields as a potential treatment for ovarian cancer after seeing the data of Novocure’s EF-14 phase 3 pivotal trial in newly diagnosed glioblastoma and learning about the treatment’s side effect profile. In Novocure’s clinical research and commercial experience to date, Tumor Treating Fields has exhibited no known systemic toxicity, with mild to moderate skin irritation being the most common side effect.
Novocure’s INNOVATE trial examined Tumor Treating Fields in combination with standard of care chemotherapy in patients with recurrent platinum-resistant ovarian cancer. The data suggested that Tumor Treating Fields in combination with weekly paclitaxel is tolerable and safe. The data also suggested more than doubling of the progression free survival and an improvement in overall survival among patients who received Tumor Treating Fields with paclitaxel compared with historical controls.
“I’ve always been very interested in innovation and trying to find new treatment modalities to help our patients,”
Based on these results, Novocure plans to start a phase 3 trial in recurrent ovarian cancer in 2018. Professor Vergote said he plans to participate.
“I’ve always been very interested in innovation and trying to find new treatment modalities to help our patients,” he said. “I’ve always done that from the start of my career.”